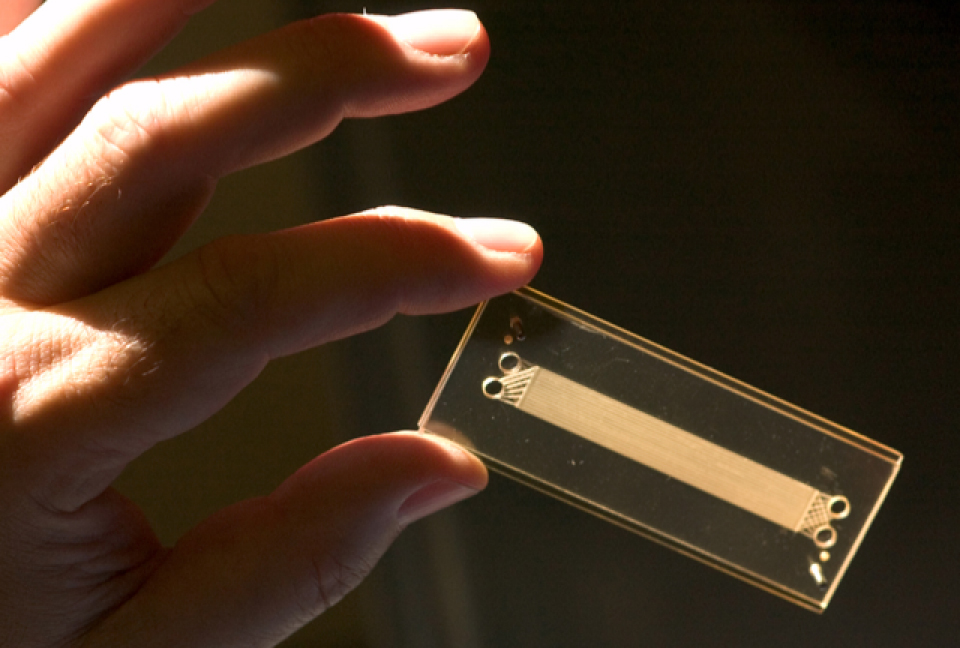
Microchannel Dialyzer
The Concept:
Current hemodialyzers employ a hollow-fiber method. Blood is passed through the fibers and dialysate flows across the fibers via a shell. Uneven distribution of hollow fibers allow for “dead” areas where mass transfer is greatly limited.
Microchannels provide a more efficient method of hemodialysis by allowing for increased control over the geometry of the system. Microchannel-based dialysis allows for much smaller units while increased efficiency reduces the large volume of dialysate waste traditionally associated with hollow-fiber dialysis. This presents opportunities for long duration treatment, home-use, and a better quality of life for patients.
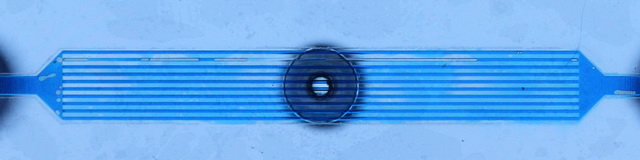
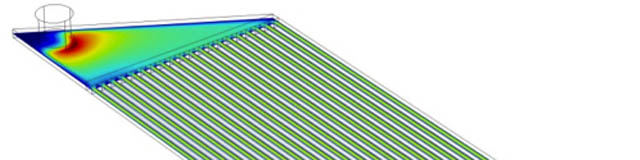
It begins with two lamina containing microchannels, separated by a dialyzer membrane. Blood flows through one set of channels and dialysate flows countercurrent on the other side. One set of these microchannels is not sufficient for proper hemodialysis. Multiple lamina can be stacked on one another and run in parallel to increase throughput.
Approaching a Solution:
The issues pertaining to bubbles exist for hollow-fiber hemodialysis too, but in general, they are usually left to do their damage. This results in even lower efficiency. We will address this bubble issue for a microchannel-based hemodialyzer with two primary methods: [1] improved fabrication methods and channel geometries and [2] microchannel surface coatings. See our articles on the publications page for current results.
Better fabrication and assembly methods such as incorporating sealing bosses, may decrease leakage and reduce channel/membrane deformation. This would prevent air from seeping into the device. Optimized channel geometries will be investigated to facilitate transport of mesobubbles through the channels and out of the system.
Microbubbles will be managed by coating the channels. Triblock copolymer coatings will be used to mask the hyrophobicity of the microchannel surface. Tethered heparin may also reduce platelet adhesion in the device, a general concern with all blood contacting devices.
The Challenges:
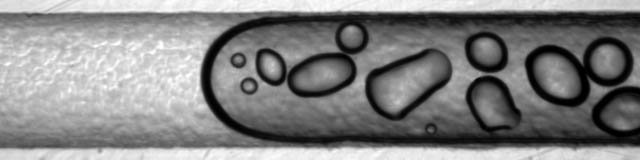
Gas bubbles present a serious challenge in design. Larger, macroscopic bubbles exist in the header regions and block off multiple channels from fluid flow. Mesobubbles exist within the microchannels, completely blocking off flow to through that channel. Both macro- and mesobubbles must be removed from the system before hemodialysis treatment can begin. Microbubbles are small enough that they do not completely block off flow like mesobubbles. They adhere to the microchannel walls and reduce efficiency. They can also cause blood damage due to high shear rates. Microbubbles are inherent to the system, especially on the blood side which contain dissolved gases and a high protein concentration. The proteins act as a surfactant, reducing surface energies and allowing for very stable microbubbles. Thus, microbubbles cannot be eliminated. Instead they must be managed.
Text by Matthew Coblyn (PhD graduate, CBEE).